York Minster is one of the largest Gothic cathedrals in Northern Europe and one of the great monuments of medieval architecture. Built in the city of York, U.K. between 1220 and 1472, it has suffered looting, vandalism, arson and a devastating fire after a lightning strike in 1984. Despite decades of restoration costing millions of pounds, the Minster still faces an implacable enemy, the air itself. In hopes of protecting the Minster from rotting away due to air pollution, Dr. Karen Wilson and Prof. Adam Lee of the Cardiff School of Chemistry, Cardiff University, along with researchers at the University of Iowa have discovered that the key to saving the church may lie in olive oil.
York Minster is made of mostly of magnesian limestone, CaxMgy(CO3)2. It’s an excellent local sedimentary stone and much used by medieval masons because of its fine-grain structure, but it’s also very susceptible to acids. Though air quality has improved over the past few decades, over two hundred years of industrial pollution is destroying local stone structures. Take a walk in the Yorkshire countryside and you’ll see how limestone field walls have been eaten away as if someone had scooped the stones out with a spoon.

A similar fate awaits the fine stonework and artistic details of York Minster. The output from factories, cars and power plants have permeated the stones with sulfur compounds and other pollutants. This became even worse over the years as repairs were made to the Minster using substandard stone with a high gypsum content. The result is that much of the stonework is blackening and corroding.
Previous solutions have involved coating the stones with hydrophobic (water repellent) coatings. The idea was to keep water out of contact with the sulfur compounds. When water comes into contact with sulfur dioxide it forms sulfuric acid, which is the key ingredient in acid rain and promotes the worst damage to the stone. The problem was that the coatings also prevented air from passing through, so pollutants became trapped and salt damage and mold growth occurred.
Recent work has concentrated on finding organic substances that would do a better job. Linseed oil has been tried in the past at York Minster, but that causes discoloration. Now the Cardiff team has hit on using a treatment that includes olive oil as a cheap, simple solution.
According the the team, the treatment consists of “naturally-sourced free fatty acids, combined with trace amounts of fluorinated alkylsilanes, which impart super hydrophobicity to pure limestone.” In plain English, that’s oleic acid and 1H,1H,2H,2H-perfluoro-decyltrimethoxysilane, C10H4F17Si(OMe). In even plainer English, that’s olive oil and a Teflon-like material that repels water, yet is permeable to air. It’s similar to the sprays used to keep rain from sticking to windscreens by means of what’s called the “lotus effect” after the way in which lotus leaves repel water. The “superhydrophobic” treatment spreads evenly over the stone and repels water with vigor, yet allows air to pass through, so the stones can “breathe.”
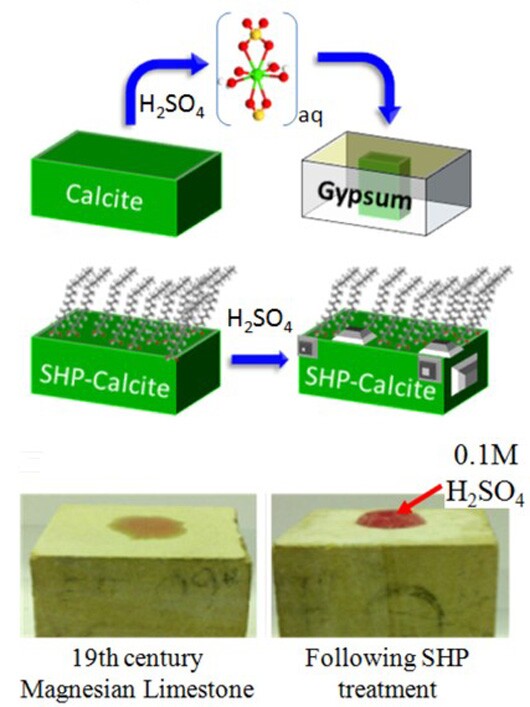
Because a mistake could cause irreparable damage that might not be discovered for decades, one vital part of the team’s work was a thorough analysis of the treatment. To test the preservation method, limestone samples were treated and then examined by X-ray absorption analysis using the synchrotron at the Diamond Light Source in Chilton. It showed that the calcium minerals in the limestone were largely protected from the acid and little gypsum was formed, as would have been expected if the stone had been attacked.
The results of the team’s work were published in Scientific Reports.
Source: Cardiff University via NPR












