Batteries
-
Natron Energy fell a little behind schedule on production plans for its sodium batteries but officially commenced production of the rapid-charging, long-life lithium-free batteries this week, bringing to market an intriguing new storage alternative.
-
China's CATL – the world's largest EV battery producer – has launched TENER, which is described as the "world's first mass-producible energy storage system with zero degradation in the first five years of use."
-
China's Betavolt New Energy Technology has unveiled a new modular nuclear battery that uses a combination of a nickel-63 (⁶³Ni) radioactive isotope and a 4th-generation diamond semiconductor and can power a device for 50 years.
-
The new Anod Hybrid bike is technically more of a 'tri-brid,' adding supercapacitors to the usual battery plus pedal power equation. This provides quick-bursting power and cuts the battery bulk down to the size of a portable power bank.
-
It never fails … you go to use a device that should be fully charged, but its battery has gone flat over time. Such may soon no longer be the case, however, if battery manufacturers simply start using a different type of adhesive tape.
-
Renewable energy grids need lots of energy storage – and EVs plugged into charging stations represent a huge, city-wide battery just waiting to help out. The Dutch city of Utrecht is about to pioneer a clever way to kickstart two-way charging.
-
Greenworks makes hundreds of indoor and outdoor power tools that run on swappable battery packs. Now the company is using those same batteries to power a new e-mobility range that will include ebikes, an electric UV and an e-minibike.
-
Although great strides are being made in the field of "smart" contact lenses, one challenge remains – how do you safely and discreetly power the things? Singaporean scientists may have the answer, in the form of a tear-fluid-charged ultra-thin battery.
-
Back in May, Ample revealed that it had managed to get its swap station to change out an electric vehicle's battery in five minutes. Now the company has partnered with Mitsubishi Fuso to bring its battery swapping technology to electric trucking.
-
Gates- and Bezos-backed startup Form Energy is one of the most exciting companies in the grid-level renewable energy storage space, with a multi-day iron-air battery system just 10% the cost of lithium. A 10-MW/1-GWh demo system has now been approved.
-
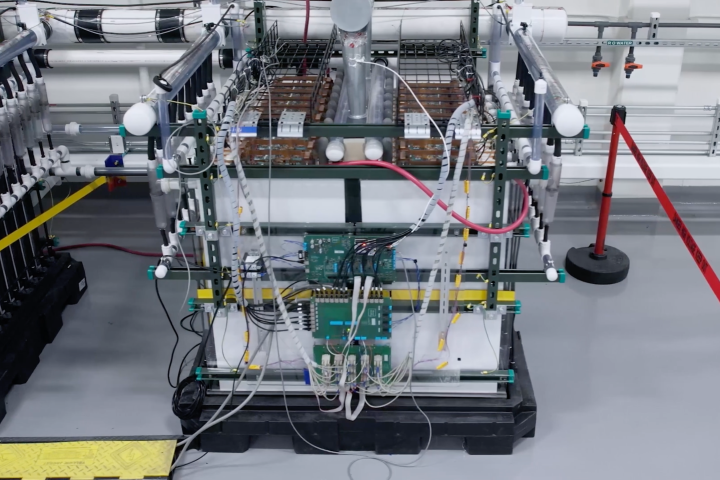
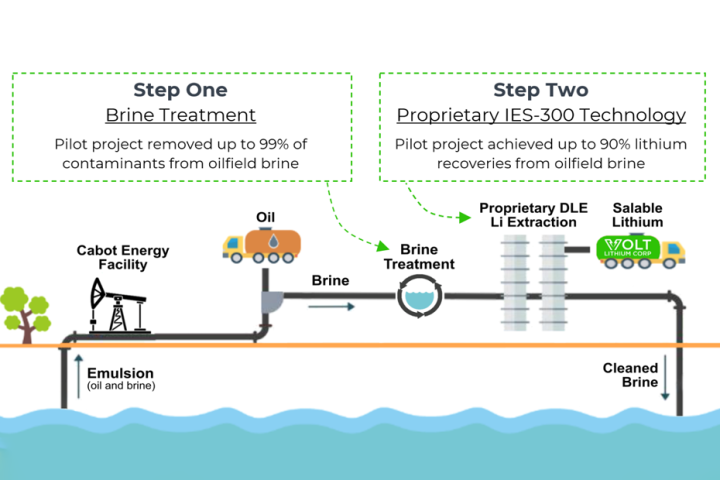
Canadian company Volt Lithium has developed and pilot-tested a new low-cost lithium extraction method to pull this critical battery metal out of low-concentration brines. Now it plans to turn old oil fields into lithium production operations.
-
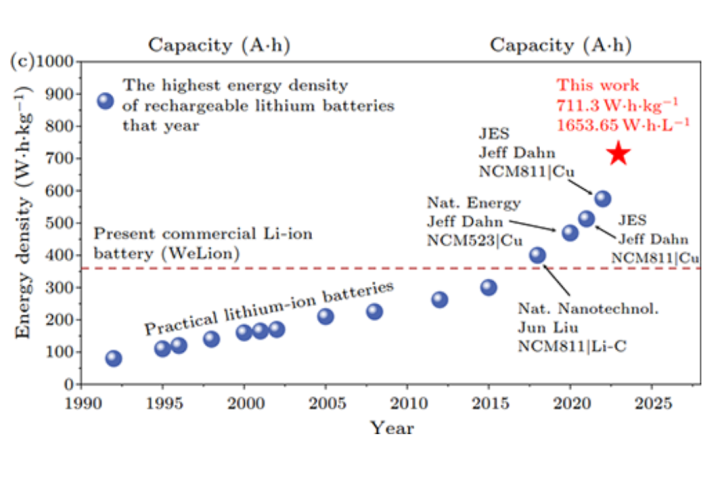
Tesla's 4680 cells, for comparison, measure somewhere between 244-296 Wh/kg. So the extreme-density cells recently tested in Beijing represent a huge leap forward from the status quo – even if they're solely focused on maximizing a single metric.
Load More