Health & Wellbeing
On these pages you'll find everything from devices designed to give you a better night's sleep to the latest work being done to tackle the obesity epidemic. Live long and prosper!
-
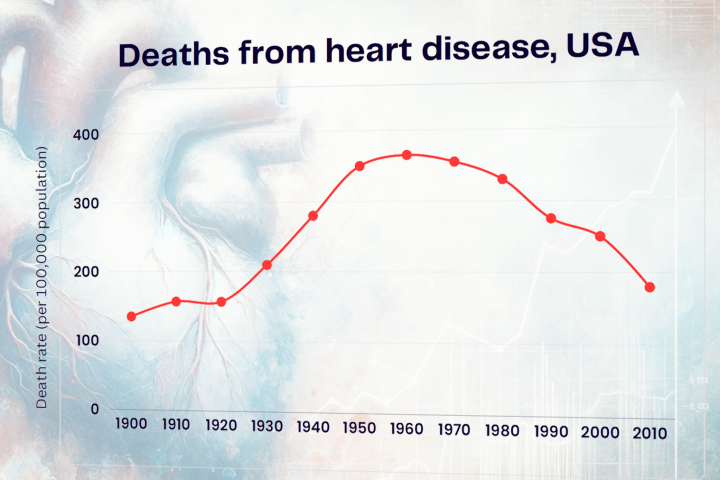
Has COVID set us up for a major heart disease epidemic? It’s happened before
April 18, 2024Researchers suggest the 1918 influenza pandemic caused a wave of heart disease in the 20th century. With new evidence showing the impact of COVID on our hearts, could this recent pandemic be setting us up for another century of cardiac problems? -
How much dietary fiber do you need to reduce high blood pressure?
April 09, 2024There's been no guide on how much dietary fiber you need to eat to reduce blood pressure – until now. A new study has confirmed that it has an effect independent of medication and quantifies just how much is needed to directly impact high blood pressure. -
Toilet flush tests reveal just how nasty it is to leave the lid up
April 07, 2024When it comes to toilet etiquette, do you put the lid down before you flush, or leave it up? A new study has shown just what happens when you leave it up – and the results might have you questioning just what you do behind closed doors. -
Watermelon overdose cases reveal a deadly risk to compromised kidneys
April 03, 202414% of American adults are affected by chronic kidney disease – and anyone in that category should be very careful about how much watermelon they eat. A new series of case studies examines how a favorite fruit can cause life-threatening issues. -
Kombucha microbes break down fat stores like fasting – without the effort
April 03, 2024It may not be to everyone's taste, but kombucha tea may be able to deliver the benefits of fasting, without the hardest part – the fasting. Its yeast and bacteria altered fat metabolism, without any other dietary changes, resulting in lower fat stores. -
Dogs can sniff trauma flashbacks on our breath
March 28, 2024Two dogs in a small study were able to correctly identify what breath smelled like when it was linked to a memory of trauma. The finding might allow dogs to be even better friends when it comes to helping PTSD sufferers cope with their conditions. -
Working-age death rate 2.5x higher in the US than other countries. Why?
March 21, 2024Americans aged 25-65 years are dying at far higher rates than their peers from other high-income countries, even surpassing death rates in Central and Eastern Europe. A new study examines what's caused the three-decade rise in midlife mortality. -
ADHD medications deliver surprise bonus benefits
March 20, 2024Living with ADHD is a complex experience, but one that's made more manageable with the right medication. What's of particular interest to researchers is how these drugs are positively impacting other mental health issues such as anxiety and depression. -
Angry? Don't go for a run, it'll just make things worse
March 19, 2024Engaging in activities that are designed to blow off steam when you’re angry probably isn’t going to be effective at reducing your anger, researchers have found. It’s better, they say, to try activities that decrease your physical arousal. -
Genetic variants link meat consumption to increased bowel cancer risk
March 18, 2024Researchers have identified two genetic markers that may explain the link between eating red and processed meat and bowel cancer. Understanding the disease process and the genes underlying it can help develop better prevention strategies. -
Asthma rates double from inner cities to outer suburbs. Here's why
March 13, 2024Combining census data with cutting-edge statistical analysis and satellite imagery, researchers have revealed a stark difference between inner- and outer-city living in terms of the risk children have of developing asthma. -
Magnetite pollution is damaging our brains and causing Alzheimer's
March 06, 2024In 2016 researchers found unusually high levels of magnetite in a number of human brain samples. The tiny toxic particle can be found in modern urban air pollution and is now suspected to be one environmental contributor to Alzheimer's disease. -
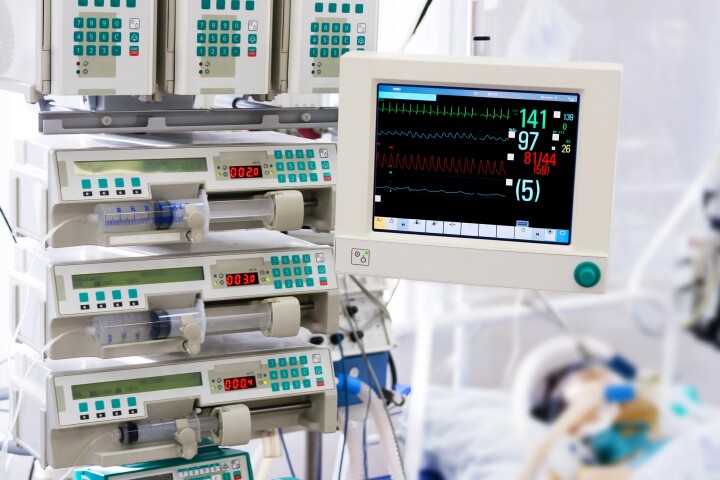
Annoying hospital beeps are causing hundreds of deaths a year
March 05, 2024Hospital workers hear up to 1,000 alarm noises per shift, and that sensory overload is costing hundreds of lives. New research suggests there's a fix that could make a significant difference – while also making key equipment far less annoying. -
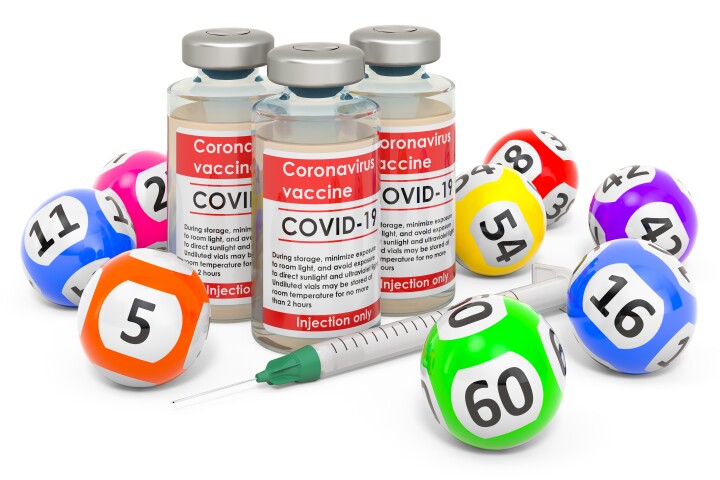
What happened to the guy who had 217 COVID vaccinations
March 05, 2024A man that police caught taking 217 doses of COVID vaccine has offered himself up to researchers for a study looking into what happens to the immune system after so many doses. The results offer surprising insight into these new mRNA vaccines. -
AI face-checking app to size up depression
February 29, 2024MoodCapture will use your smartphone camera as you unlock the screen to assess facial expressions and backgrounds for depression severity. While the app is still in development, the team says it represents a breakthrough in personal diagnostic medicine.
Load More