Researchers at the University of California, Riverside have developed a new graphene-based supercapacitor that uses a nanoscale architecture to double its energy and power performance compared to commercially-available alternatives. This breakthrough is another important step toward making supercapacitors viable for use in fast-charging, high-performance electric cars and personal electronics.
Supercapacitors are energy storage devices that are very stable and long-lived, with high power density (power per unit of volume) but very low specific energy (energy stored per unit of mass). This means supercapacitors are able to deliver large amounts of power, but only for a few seconds at a time.
A team of researchers led by Prof. Cengiz S. Ozkan at UCR has now developed a new design for a supercapacitor with a specific energy of 39.3 Wh/kg and power density of 128 kW/kg, which is roughly double the performance of commercial supercapacitors in both these respects.
The researchers achieved this result by developing a new 3D carbon nanotube porous foam. The nanoscale pores provide a very large surface area that facilitates the infiltration of electrolyte and allows it to store energy much more densely than in conventional designs.
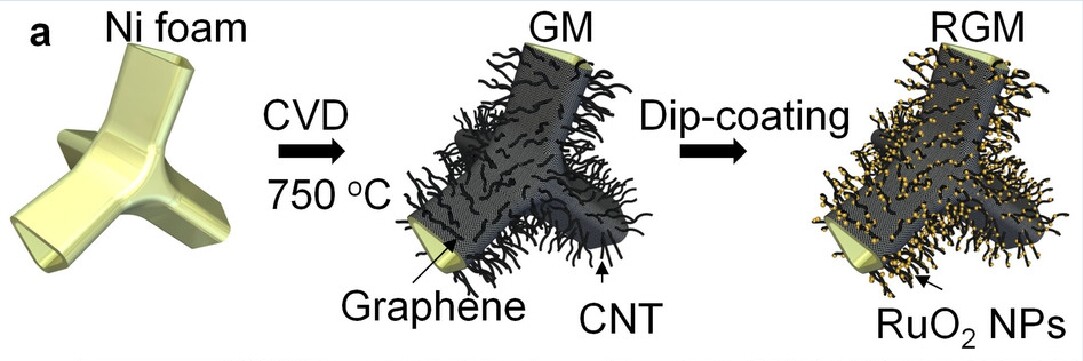
The foam was prepared by chemical vapor deposition of graphene and carbon nanotubes over a nickel substrate (see illustration above), and successive deposition of nanoparticles of hydrous ruthenium oxide (RuO2), where each particle was under five nanometers in size. Inside the foam, graphene acts as both a current collector and a buffer layer to facilitate the conduction of electrons and insulate the foam from the electrolyte.
One of the main strengths of supercapacitors over batteries is their superior cycling performance, and this device is no exception. Curiously, its capacitance (the capacity of the device to hold an electric charge) not only stabilized, but actually improved by 6 percent after 8,100 charge-discharge cycles. The researchers believe that this is due to the electrochemical activation of the active material.
The excellent performance, stability and ease of preparation of this system make it an attractive solution for future mass-production. And although its specific energy has ways to go to catch up with lithium-ion battery technology, it is certainly an important step in the right direction.
An open-access paper describing the advance was published on the journal Nature Scientific Reports.
Source: UC Riverside






