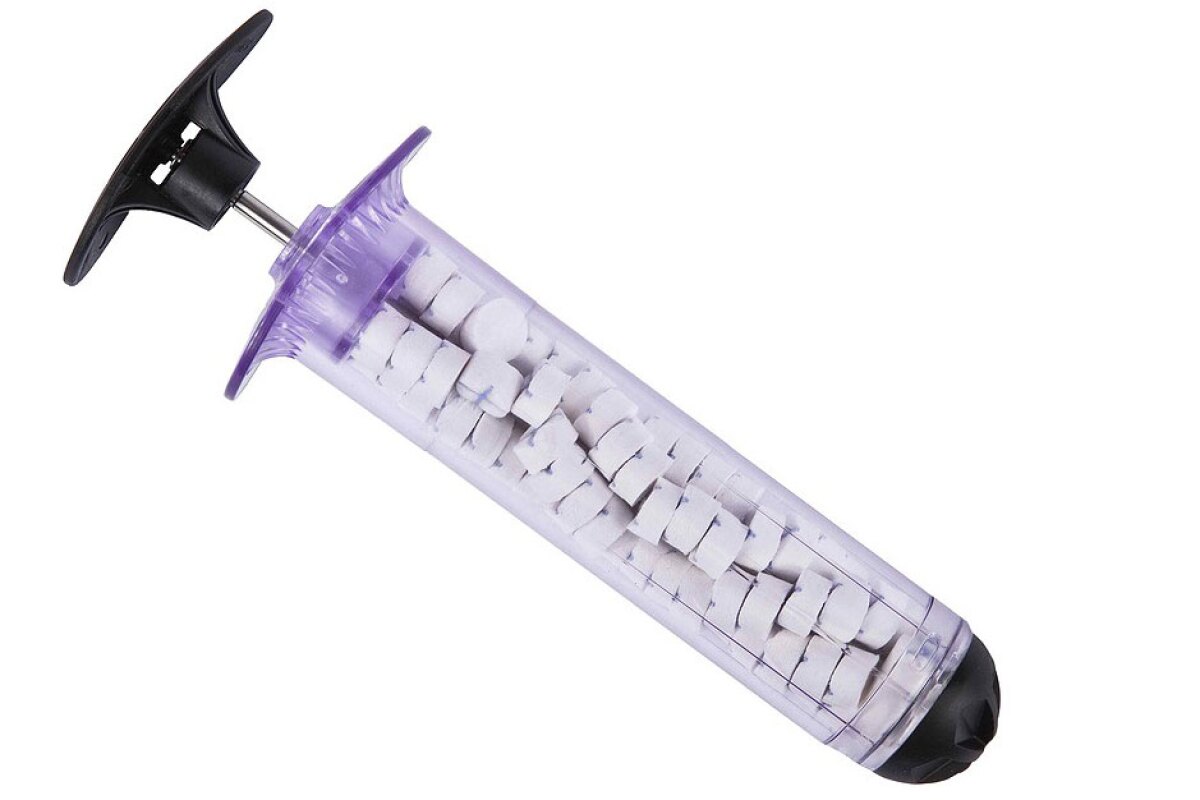
A fast-acting medical device developed for emergency treatment of a gunshot or other penetrating wound on the battlefield has been cleared for civilian use by the US Food and Drug Administration (FDA). The XStat 30 is a plastic syringe that can stop severe bleeding within around 20 seconds through the injection of small sponges into a wound, and will now be available for use by the general population.
Taken from prototype stage through to its final development under a US$5 million US Army contract, the first variant called XStat was approved for use on the battlefield in 2014. Narrow, penetrative wounds like those resulting from a gunshot are obviously very agonizing, but emergency treatments in the field involving the application of special gauze and direct pressure cause extreme discomfort for the patient, too.
Functioning in essentially the same way as the military version, XStat 30 works by filling a wound with small cellulose sponges, which are made from wood pulp and coated in chitosan, a compound found in crustacean shells. Researchers have previously sought to harness the antimicrobial properties of chitosan for things like tackling superbugs and spray-on coatings that boost the shelf life of fresh fruits.
In this case, further to its bacteria-battling abilities, chitosan also serves to trigger clot formation. This works in conjunction with the expanding sponges to fill the cavity, apply sufficient pressure to stop arterial bleeding and quickly stem blood flow. And as the sponges also contain an x-ray absorbing material, they can then be detected and safely removed when surgical treatment later becomes available.
The dressing can be used for up to four hours and is intended for life-threatening, non-compressible junctional wounds when care at an emergency facility is not readily available. It is not meant for use in parts of the chest, abdomen. pelvis or tissue above the collarbone. Each syringe contains 92 sponges and can absorb around a pint (473 ml) of blood, and up to three syringes can be used on each patient.
The US Army Institute of Surgical Research says that 30 to 40 percent of civilian deaths by traumatic injury stem from haemorrhaging, of which between 33 and 56 percent take place before the patient even reaches the hospital.
"It is exciting to see this technology transition to help civilian first responders control some severe, life-threatening bleeding while on the trauma scene," says William Maisel, acting director of the Office of Device Evaluation in the FDA's Center for Devices and Radiological Health.
Source: FDA