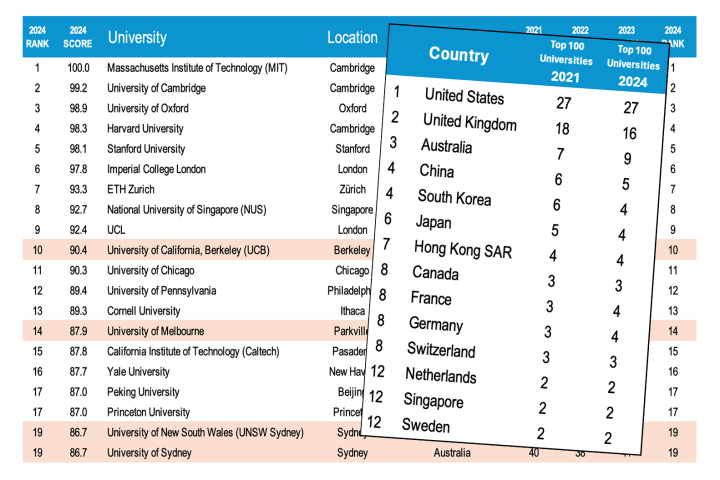
In their continuing efforts to increase the energy density of lithium-ion batteries, scientists have began looking at alternative materials for those batteries' electrodes – materials such as silicon. The problem is, electrodes swell and shrink as they absorb and release lithium ions, causing them to break down over time. This is particularly true of silicon, which is brittle by nature. Now, however, scientists have developed a conductive elastic polymer coating for those electrodes, that heals its own cracks after each use.
The polymer was created by a team from Stanford University and the Department of Energy’s SLAC National Accelerator Laboratory, in the lab of Stanford's Prof. Zhenan Bao. It incorporates an elastic material that Bao and colleagues had already developed for use in skin for robots and prosthetic limbs. Carbon nanoparticles were added for this application, to make the polymer electrically conductive.
When silicon electrodes were coated with the material, it held them together throughout their swelling and shrinking cycles a reported ten times longer than when they were left uncoated. Small cracks did appear in the polymer itself, but those closed up within a few hours of forming.
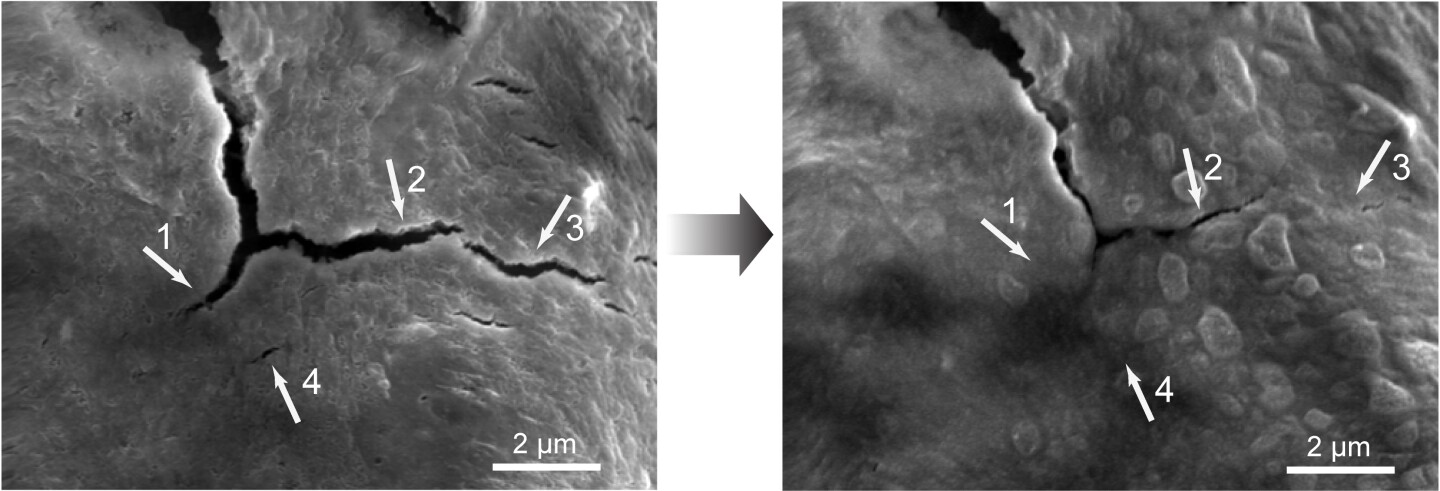
The self-healing quality of the material was made possible by deliberately weakening some of the existing polymer's chemical bonds. This actually made it easier to break, but also caused the edges of those breaks to be chemically drawn back together, linking up as they met. Biological molecules such as DNA utilize a similar process.
In lab tests, the coated silicon electrodes lasted for about 100 cycles. "That’s still quite a way from the goal of about 500 cycles for cell phones and 3,000 cycles for an electric vehicle, but the promise is there, and from all our data it looks like it’s working” says Prof. Yi Cui, who led the research along with Bao.
What's more, the scientist believe that the polymer could also be used with electrodes made from materials other than silicon.
A paper on the research was recently published in the journal Nature Chemistry.