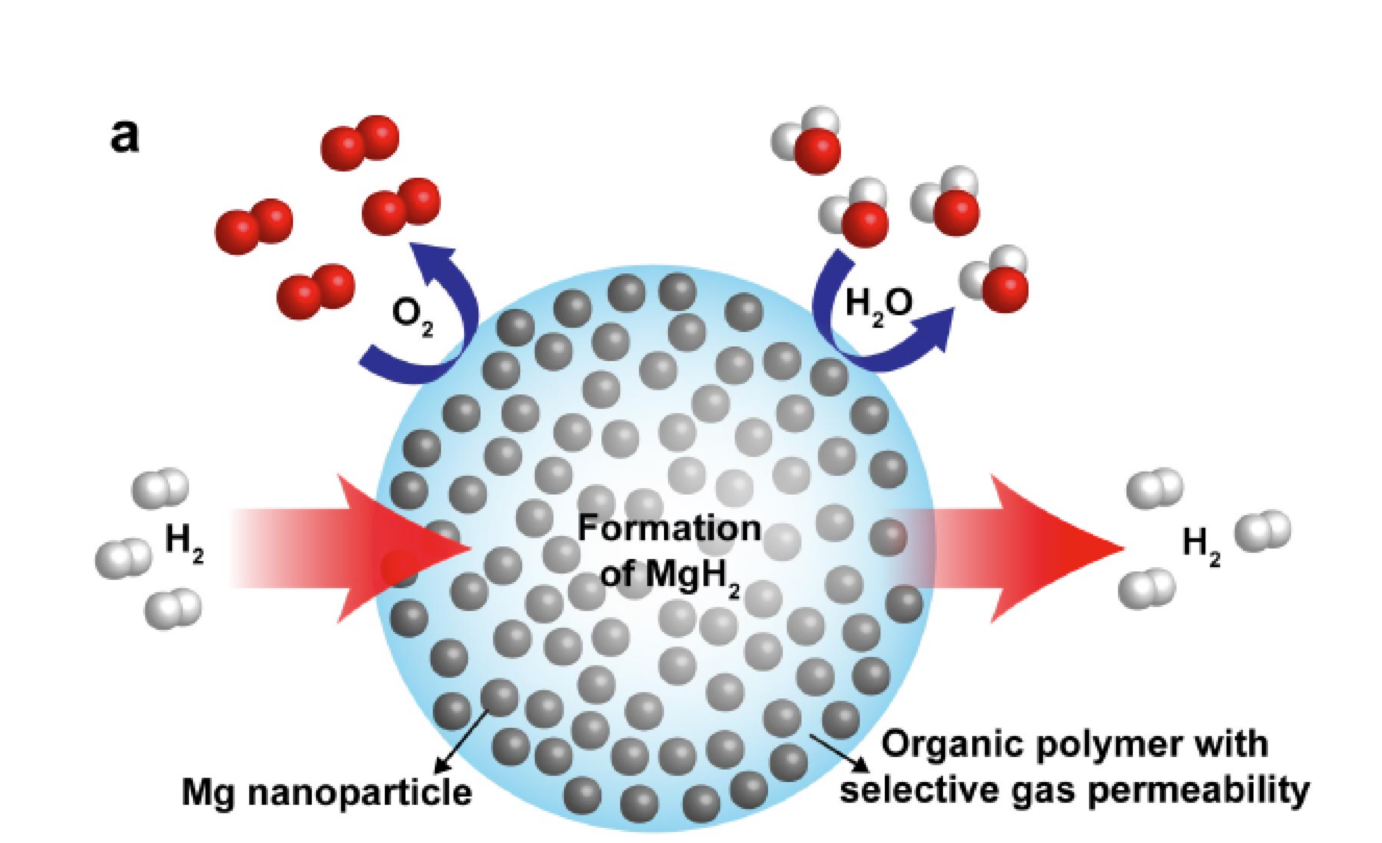
Scientists from the U.S. Department of Energy's Lawrence Berkeley National Laboratory have created a composite material that they claim can store hydrogen densely and safely, yet that also allows it to be easily accessed for creating electricity. Some materials that are currently used for hydrogen storage have a relatively small capacity, and need to be superheated or supercooled in order to work at peak efficiency. The new material, however, is said not to have either of these limitations.
The Berkeley Lab researchers created the pliable nanocomposite from a matrix of polymethyl methacrylate, which is a polymer related to Plexiglas, with nanoparticles of magnesium sprinkled throughout. It reportedly is able to absorb and release hydrogen at "modest temperatures," without oxidizing the magnesium after cycling.
To confirm that hydrogen was present within the magnesium, the researchers observed the nanoparticles through the world's most powerful transmission electron microscope, the TEAM 0.5 – also located at Berkeley Lab.
"This work showcases our ability to design composite nanoscale materials that overcome fundamental thermodynamic and kinetic barriers to realize a materials combination that has been very elusive historically," said Jeff Urban, Deputy Director of the Inorganic Nanostructures Facility at Berkeley Lab's Molecular Foundry. "Moreover, we are able to productively leverage the unique properties of both the polymer and nanoparticle in this new composite material, which may have broad applicability to related problems in other areas of energy research."
The team's findings were recently published in the journal Nature Materials.






