Scientists at the National Institute of Standards and Technology (NIST) and the National Institutes of Health (NIH) have developed a new type of shape-shifting nanoprobe that can perform high-resolution remote biological sensing not possible with current technology. Around one-tenth the size of a single red blood cell, the nanoprobes are designed to provide feedback on internal body conditions by altering their magnetic fields in response to their environment. The researchers predict wide-spread applications for the nanoprobes in the fields of chemistry, biology, engineering and, one day, to aid physicians in high-accuracy clinical diagnostics.
Dubbed geometrically encoded magnetic sensors (GEMs), the nanoprobes are microengineered from two plates of magnetic metal disks 0.5 to 2 micrometers in diameter and just tens of nanometers thick. These are formed either side of a polymer gel to create a microminiature sandwiched component.
More specifically, the polymer is a layer of hydrogel, a network of polymer chains that are hydrophilic (absorb water) and are able to expand significantly dependent upon the level of the moisture in the environment in which they are used. Similarly, the gel can also contract when the environment is low in moisture. As such, the expanding or contracting of this gel then changes the distance between the two magnetic disks, and in turn increases or decreases the magnetic field.
This change in magnetic field strength then affects the resonant frequency of the protons contained in water molecules in and around the gel in response to applied radio-frequency radiation. As a result, scanning the environment with a set of different frequencies allows rapid identification of the shape of the nanoprobes at that time. This then allows determination of the remote conditions being measured.
The over-riding advantage to this sort of nanoprobe measurement is that it uses RF energy to observe conditions within the body to greater depths and with higher resolution and sensitivity. Many existing imaging technologies looking at biochemical environments such as abnormal acidity or alkalinity and ion concentration rely on a range of nano-sized sensors that are operated and assessed using light at visible frequencies. However the resolution of the these optical signals decreases markedly in relation to their depth within the body under examination, thereby limiting their use to easily accessible regions.
According to the research team, the magnetic disk/hydrogel combination is not limited to easily accessible or shallow areas, thereby making possible the real time analysis of environments on a molecular scale buried deep inside tissue structures.
"Our design is based on completely different operating principles," said Dr Gary Zabow, senior research fellow at NIST. "Instead of optically based sensing, the shape-changing probes are designed to operate in the radio frequency (RF) spectrum, specifically to be detectable with standard nuclear magnetic resonance (NMR) or magnetic resonance imaging (MRI) equipment. In these RF ranges, signals are, for example, not appreciably weakened by intervening biological materials."
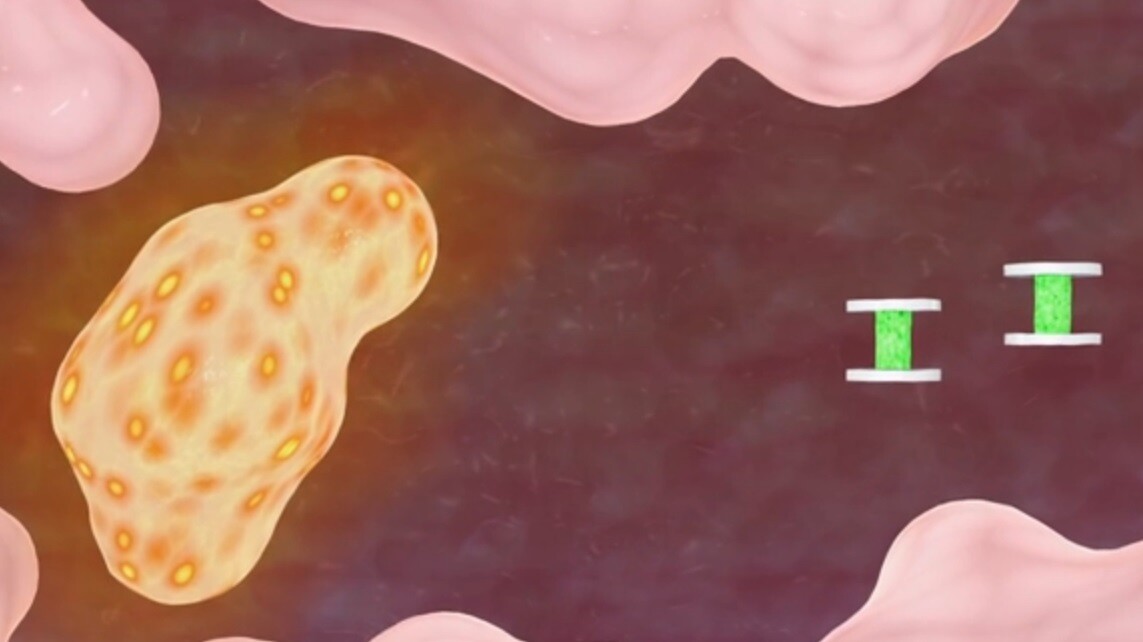
The scientists have tested the nanoprobes in various solutions, including those of varying pH, ion concentration, and in a growth medium liquid populated with living kidney cells, observing their changes in metabolism as they transitioned from functioning to non-functioning due to a lack of oxygen. In this test, the reaction of the cells increased the acidity level of the growth medium and the change was recorded through real-time shifting in resonant frequencies by the GEMs implanted in the medium.
In addition, the researchers assert, the basic, first-generation probes used in these early trials were easily able to resolve frequency shifts resulting orders of magnitude better than any comparable frequency shifting observations collected through currently available magnetic resonance spectroscopy methods.
While not the first nanoprobe to change state due to a change in the biological environment in which it is situated – a graphene-clad "cytobot" from the University of Illinois at Chicago being one notable example – the GEM nanoprobes are able to directly indicate a specific pH state rather than simply a change in humidity.
As such, given that the measurement of specifically-located pH values within a living body is an exceptionally difficult thing to achieve, particularly as such things as blood tests are simply unable to draw highly-localized samples as they are too large and too great a mix of surrounding liquids. However it is an important and valuable capability of detecting pH in specific areas that can be of significant help in early diagnosis of certain diseases, for example in the indication of an unseen tumor.
"Of course, that sort of potential use in living organisms is still a long way off," said Dr Zabow. "Our data were taken in vitro. And some potential applications of the sensors may not be biological at all. But a long-term goal is to improve our techniques to the point at which GEMs can be employed for biomedical uses."
To be able to suit such important biological applications, however, greater reductions in size to somewhere in the region of less than 100 nanometers in diameter would be needed to allow the probes access to the generally smaller structures that make up a great deal of the human body.
The current raft of GEMs are also able to be adjusted during their creation to respond to varying biochemical conditions by being tailored to resonate at different frequencies, dependent upon the the gel composition and magnet shapes and materials used. This, then, makes it possible to insert differently structured nanoprobes in the same localized environment and – by detecting their responses at different frequencies – makes it possible to detect and measure two separate variables at once.
The researchers say they have already demonstrated this ability by scanning a medium with two dimensionally-distinct GEMS and detecting the signals from both at the same time.
"The idea is that you could design different sensors to measure different things, effectively measuring a panel of potential biomarkers simultaneously, rather than just one, to better differentiate between different pathologies," said Dr Zabow. "We think that these sensors can potentially be adapted to measure a variety of different biomarkers, possibly including things such as glucose, local temperatures, various ion concentrations, possibly the presence or absence of various enzymes and so forth."
Future work by the team is aimed at both further miniaturization, along with a method to create and tailor large numbers of these shape-shifting nanoprobes for release in commercial quantities and eventual use in future biomedical sensing techniques.
"The work on geometrically encoded magnetic sensors by Gary Zabow and colleagues is a natural extension of research published by the team, along with NIST's John Moreland, in 2008. That work showed how micromagnets can act as 'smart tags' to potentially identify particular cells, tissues or physiological conditions," said Ron Goldfarb, leader of NIST's Magnetics Group. "Functionally, the GEMS in the current effort are more advanced in that they change their shape in response to stimuli; thus, they act as measurement devices. The next challenge will be design optimization and the development of dimensionally controlled, large-scale fabrication processes in order to make these sensors widely available to researchers."
The short video below shows an illustrated depiction of the nanoprobes in action.
The results of this research were recently published in the journal Nature.
Source: NIST






